Are prescription drugs becoming more dangerous?
According to USA Today, 2022 saw the highest number of drug recalls in the past three years for human drug products – 1321. That’s more than 1300 drug products that the FDA determined were either violating laws or defective.
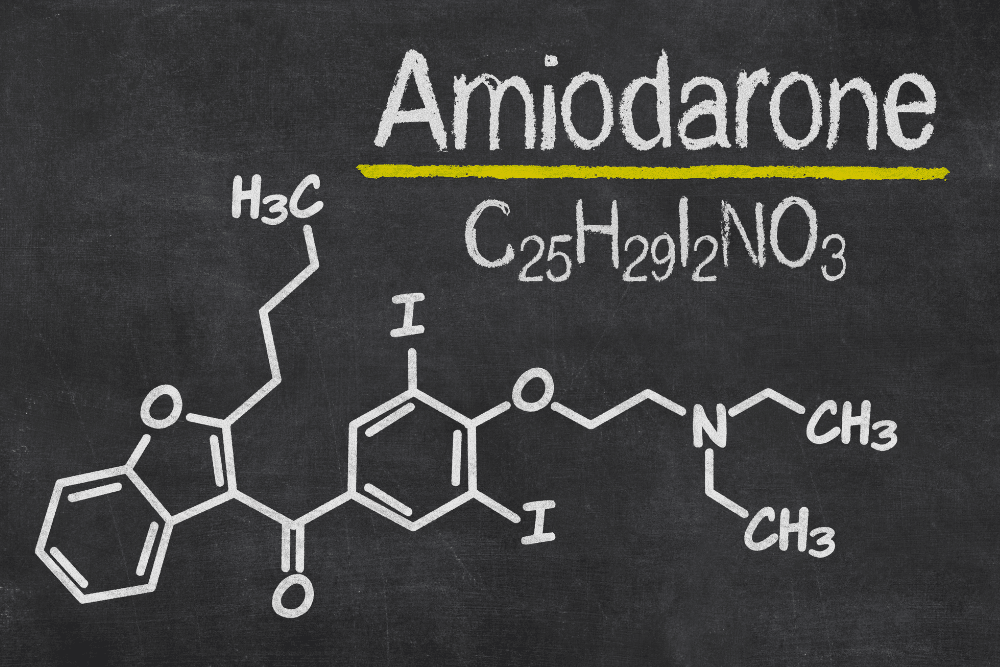
This isn’t even counting drugs that cause serious problems but still haven’t been recalled. One of the most problematic of these is amiodarone.
Amiodarone is a medication used to treat heart arrhythmias, a condition in which the heart beats irregularly. It was first approved by the FDA in 1985, at a time when there were few other treatments available for arrhythmias.
In fact, this lack of other options is what led the FDA to approve the drug despite it coming with a host of potentially life-threatening side effects, including lung damage, liver damage, thyroid problems, and vision changes – including the potential for blindness. There have even been cases that resulted in death for the victim.
Originally, amiodarone was only intended as a drug of last resort for patients who had not responded to other medications or procedures. However, over time the manufacturers of amiodarone were able to create a bigger market for the drug by aggressively marketing it to doctors and downplaying its risks – something that has led to injury for countless people who arguably never should have been taking the drug in the first place.
How was the manufacturer able to do this? And what can you do if you or a loved one were injured?
Expanding the Market for a Dangerous Drug
One of the ways the manufacturers of amiodarone were able to create a bigger market for the drug was through aggressive marketing tactics. They promoted the drug as a highly effective treatment for cardiac patients, even though its risks were already well known.
In fact, according to a report from the U.S. Department of Justice, “aggressive” isn’t a strong enough term. The DOJ found that the manufacturers of amiodarone engaged in a variety of illegal marketing tactics, including paying doctors to promote the drug, giving doctors expensive gifts and meals in exchange for prescribing it, and sponsoring educational seminars that promoted the drug to doctors.
What specifically did the manufacturer do?
They downplayed its risks. According to a report from the Institute for Safe Medication Practices, the manufacturers of amiodarone failed to warn doctors and patients about the drug’s potential side effects, including its tendency to cause lung damage, liver damage, thyroid problems, and vision changes.
Instead, they focused on the drug’s efficacy in treating heart arrhythmias and presented it as a “miracle drug” that could save lives. This led many doctors to prescribe amiodarone without fully understanding the risks, and many patients to take it without fully understanding the potential consequences.
They used their lobbying power. The lobbying power of big pharmaceutical companies is a well-known and ongoing problem in our country. Politicians and others have been speaking out against it for years, but no matter who’s in office, nothing seems to really change.
In the case of amiodarone, not only did the manufacturer lobby for the drug to be covered by insurance plans, they pushed for expanded indications, meaning it could be used for more than just its originally approved purposes.
They funded research and clinical trials. One method drug companies use to show a drug’s efficacy and safety is to put it through clinical trials. However, in the case of amiodarone, the manufacturer worked to create a bigger market for the drug by funding studies that specifically supported its use. These studies were often carefully designed to show that amiodarone was more effective than other treatments, or that its benefits outweighed its risks.
By itself, that wouldn’t have been horribly damning. After all, all pharmaceutical companies have a vested interest in showing that their product works better than others, and it’s not uncommon for them to pay for their own studies. However, outside investigations discovered that many of these studies were flawed, and some were later found to have been manipulated or outright falsified.
What happened? In short, all the campaigning worked. Between 1987 and 2001, amiodarone use increased by more than 500%. Many of the patients using it probably had the option for less dangerous alternatives, but these were not being pushed as hard. Unsurprisingly, a significant number of people taking the drug suffered serious injuries.
An Explosion in Use Led to a Huge Uptick in Injuries – and Corresponding Lawsuits
In recent years, as a number of those off-label users suffered injury due to using amiodarone, there have been a number of lawsuits filed against the drug’s manufacturers. These lawsuits allege that the drug caused serious harm to patients and that the manufacturers failed to adequately warn patients and physicians about the risks. In other words, they should be held liable for any damages that people have suffered due to amiodarone due to those negligent actions.
Many of the suits have been successful, and the manufacturers have been ordered to pay large settlements to plaintiffs. Moreover, a number of amiodarone lawsuits are still ongoing. Despite these legal challenges, amiodarone remains on the market, and it continues to be prescribed to patients with heart arrhythmias.
If you or someone you love has been injured through the use of amiodarone, you may be entitled to compensation for the damages you’ve suffered. These types of cases tend to be incredibly complicated, so your best bet at holding negligent parties accountable and receiving the compensation you need and deserve is to work with a NY injury attorney who has a track record of success.